Is Hydrogen Negative or Positive in H2o
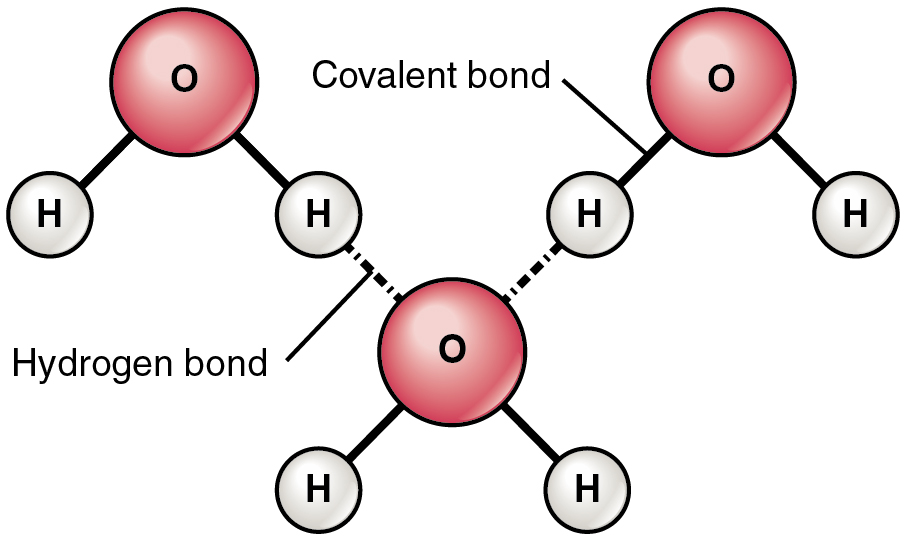
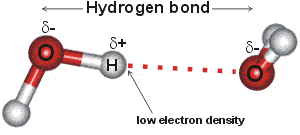
Individual hydrogen protons rarely exist freely in solution. When a hydrogen atom is coupled to a highly electronegative atom the shared pair of electrons are attracted more by this atom and the molecules negative end becomes slightly negative while the positive end becomes slightly positiveThe negative end of one molecule attracts the positive end of the other resulting in the formation of a weak bond.
Does Water Form Hydrogen Bonds With Other Water Molecules Quora
Although hydrogen bonding is a relatively weak attraction compared to the covalent bonds within the.

. The charge differences cause water molecules to aggregate the relatively positive areas being attracted to the relatively negative areas. Each reaction chamber requires 2 Volts that is why a 12 Volt car is limited to seven plates cell. But this is not guaranteed as.
Every individual cell still functions at 12 V. Hydrogen sulfide is more and more danger thann sulfur dioxide. What is the polarity.
However hydrogen is also a short-lived indirect greenhouse gas that can easily leak into the atmosphere. HCL is neither polar or non-polar. 1 Cr2O72- H e- Cr3 H2O 2 S2- I2 I- S Redox Reaction is a chemical reaction in which oxidation and reduction occurs simultaneously and the substance which gains electrons is termed as oxidizing agent.
Table of Common Ions. The seven plate design forms six electrolysis reaction chambers where there is one positive one negative positive and five neutral plates. What is the oxidation number of sulfur in h2s.
Low metabolic rate is a pro-viral influence so H2O2 might show antiviral effects. This attraction hydrogen bonding explains many of the properties of water such as its solvent properties. A charge is a property of matter that is used to classify whether a thing is positive or negatively charged.
When hydrogen atoms are joined in a polar covalent bond with a small atom of high electronegativity such as O F or N the partial positive charge on the hydrogen is highly concentrated because of its small size. So hydrogen should have positive oxidation number and sulfur has a negative oxidation number. First identify which atom has the higher electronegativity.
Hydrogen forms positive ions and oxygen forms negative ions. Like magnets alike charges repel and opposites attract. It is the.
The positive electrode is known as the anode while the negative one. Water is composed of the molecule H2O which stands for 2 atoms of Hydrogen and 1 atom of Oxygen bonded together. We use this to our advantage by using an electric field to pull the water molecules apart.
If the hydrogen is close to another oxygen fluorine or nitrogen in another molecule then there is a force of attraction termed a dipole-dipole. In ionic compounds hydrogen can take the form of a negative charge ie anion where it is known as a hydride or as a positively charged ie cation species denoted by the symbol. Hydrogen plays a particularly important role in acidbase reactions because these reactions usually involve the exchange of protons between soluble molecules.
Hydrogen is considered a key strategy to decarbonize the global economy. Although the equilibrium constant is measured when a reaction is at equilibrium this does not mean that all reactions are allowed to proceed to equilibrium. Secondly all atoms to which hydrogen is attached are not only negative but that each element should have one active lone pair present in the outermost shell.
We know that the oxygen atom and hydrogen atoms. In the cell many reactions are constantly resupplied with various chemicals which. Valency 1 Valency 2 Valency 3.
By placing two electrodes metal plates into water we can create an electric field between them by connecting them to the terminals of a battery or power supply. Food grade hydrogen peroxide can come in strengths varying from 3 to 35. Sulfur and hydrogens electronegativity values are 25 and 21 respectively.
So youd have to use 10-12 drops of 3 before youd get to the same level as one drop of 35. In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment with a negatively charged end and a positively charged end. Plate dry cells are simply several 6-chamber cells connected together to form one unit.
We can illustrate this by using water. And now it could just grab any hydrogen proton but probably the most convenient one would be this one because if this nitrogen is going to use this lone pair to form a bond with carbon its going to have a positive charge and it might wanna take these electrons back. Refer the following table which gives you oxidation numbers.
Thus it attracts electrons to spend more time at its end giving it a negative charge and hydrogen a positive charge. Hydrogen peroxide is a general stimulant to metabolic rate. So you could imagine where one of these lone pairs is used to grab this hydrogen proton and then the.
Firstly Hydrogen is attached to one of most electronegative elements and this bonding causes hydrogen to acquire a positive charge. H2O Auto Ionization Cells Free Energy and the Equilibrium Constant. Given that the climate impacts from hydrogen leakage have not been well understood especially in the near-term we assess impacts over all timescales for plausible leak rates.

Question Video Stating How Many Hydrogen Bonds Can Be Formed By A Single Water Molecule Nagwa

Sharp S Plasmacluster Technology Positive And Negative Ions Purify The Air Try It For Bett Ionic Air Purifier Air Purifier Humidifier Portable Air Purifier

1st Gen Spe Pem Portable Hydrogen Generator Water Bottle Usb Rechargeable Ionizer 14 Oz In 2022 Hydrogen Generator Alkaline Water Ionizer Bottle

Hydrogen Bonds In Water Article Khan Academy

Hydrogen Bonding Gives Water Its Unusual Properties
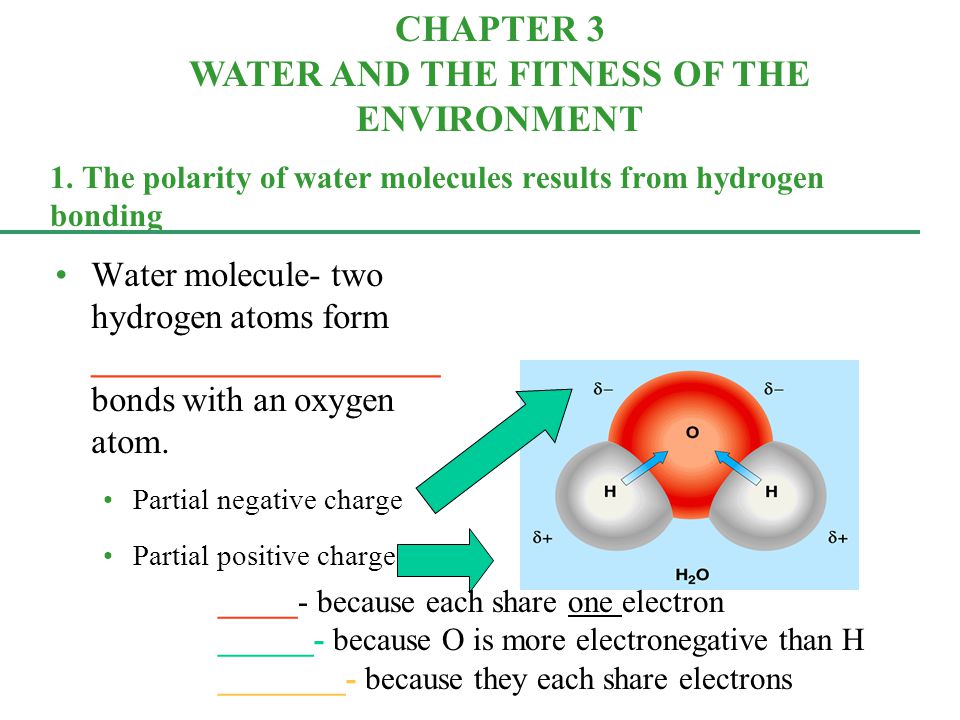
1 The Polarity Of Water Molecules Results From Hydrogen Bonding Water Molecule Two Hydrogen Atoms Form Bonds With An Oxygen Atom Ppt Download

Dipole Moment Of Bef2 Covalent Bonding Chemical Bond Ionic Bonding

Density Miscibility Lesson Hydrogen Bond Chemistry Lessons Water Molecule
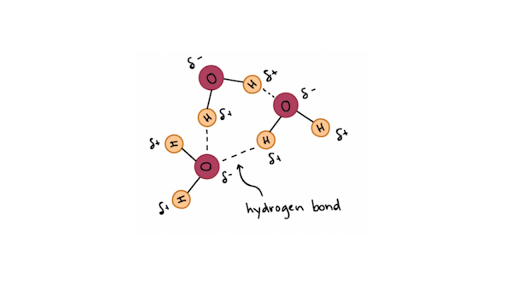
Hydrogen Bonds In Water Article Khan Academy
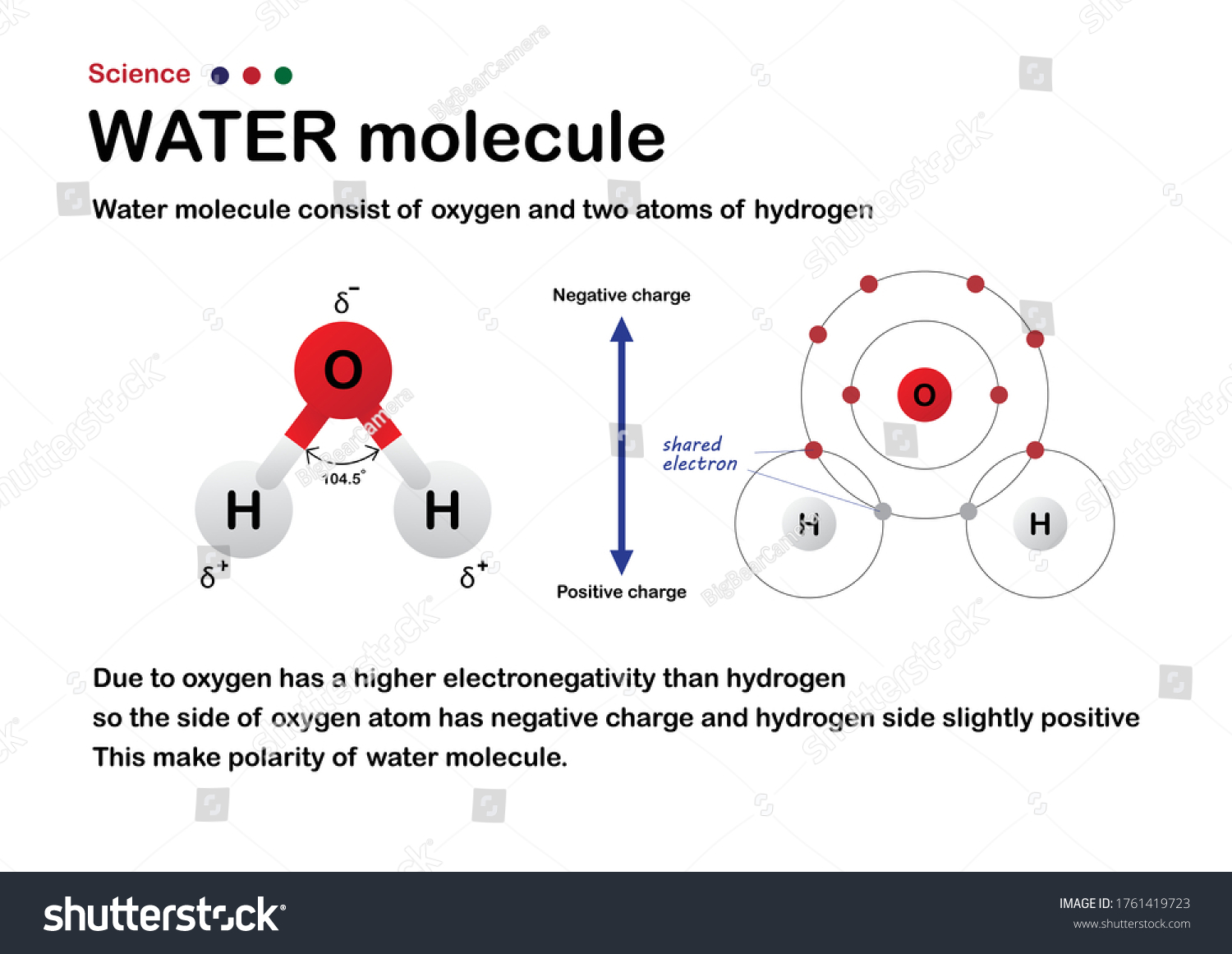
Scientific Diagram Show Water Molecule Oxygen Stock Vector Royalty Free 1761419723 Shutterstock

Molecular Composition Of Water Chemical Structure Biodynamizer

Water Molecules Poster Zazzle Com Water Molecule Education Poster Classroom Posters

Watermoleculeh2ox72 Feria Cientifica Educacion Cientificos
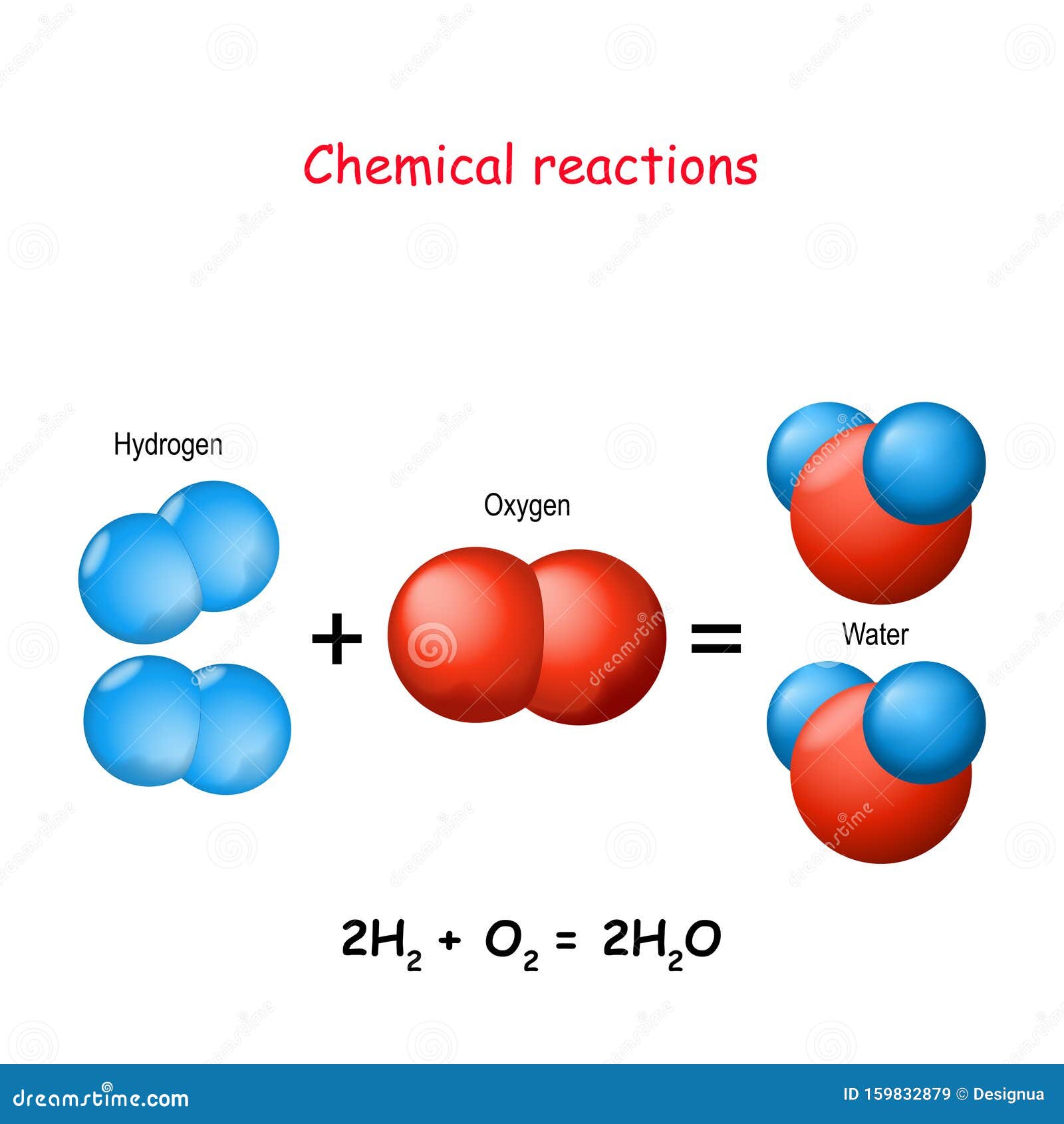
Water Molecule Oxygen And Hydrogen Stock Vector Illustration Of Change Aqua 159832879

Polarity Of Water Molecules Infographic Diagram Showing Its Microscopic View Along With Crystal Structure Of Salt An Polarity Of Water Water Molecule Molecules
Topic 2 2 Water Amazing World Of Science With Mr Green

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

Comments
Post a Comment